Under Which Conditions Will Gases Best Dissolve in Liquids
XY is a polar gas dissolved in a nonpolar solvent XY is a nonpolar gas dissolved in a polar solvent XY is a polar gas dissolved in a polar solvent The. And you can demonstrate this every time you take the cap off a bottle of fizzy or carbonated drink.

Gas Solubility And Temperature Introduction To Chemistry
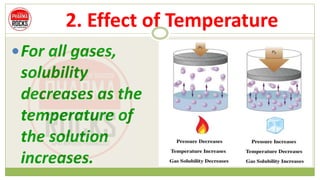
Solubilities of Gases in Water Methane oxygen carbon monoxide nitrogen and helium all have different solubilities in water but all of them become less soluble with increasing temperature.

. Gases best dissolve in liquids when the liquid temperature is low and when pressure is increased. Certainly a soft drink usually sealed under pressure will foam more vigorously when it is at room temperature than when it is chilled. The number of gas molecules.
The amount of gas that can be dissolved in water depends on the temperature of the water. So gases dissolve must easily in solutions that are under pressure. High pressure to force the gas into the liquid and low temperature so that the moving gas particles have less resistance to.
Under which conditions will gases best dissolve in liquids. The dissolving of a gas in water depends on the interaction between the molecules of the gas and the water molecules. Chemistry 22112019 1231 kaileyy06.
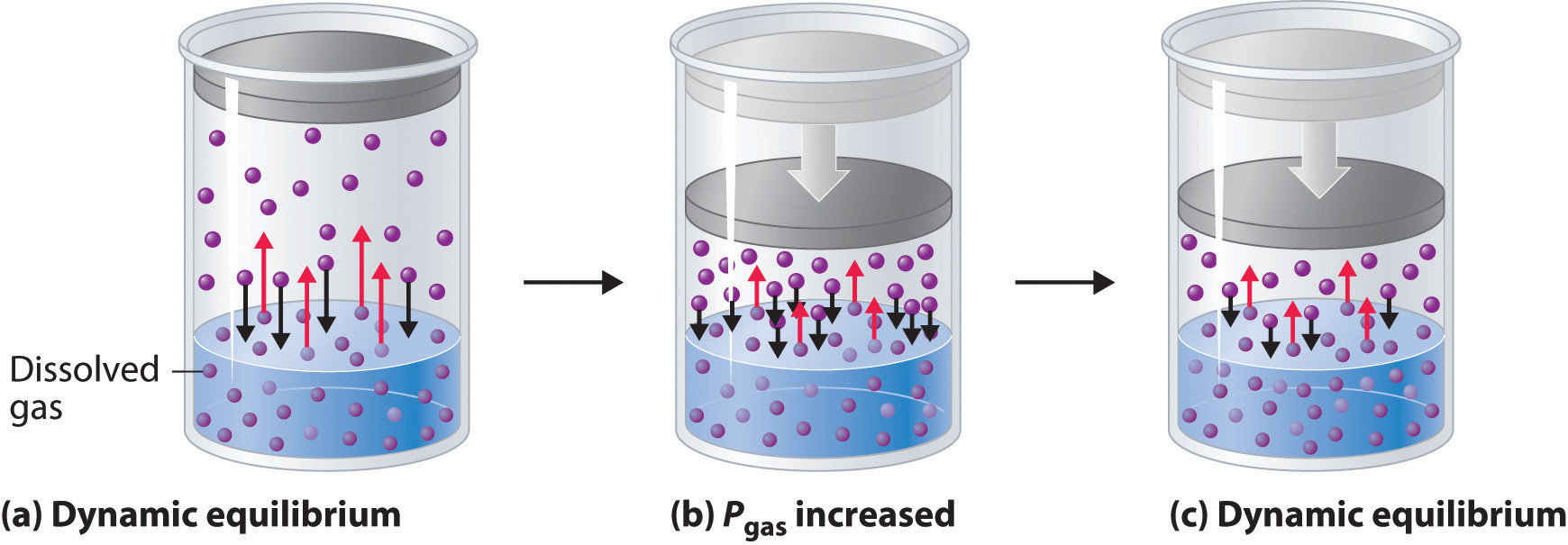
Under which conditions will gases best dissolve in liquids. This shows that gases dissolve best in a cold solution not in a warm solution. The solubility of a gas in a liquid is directly proportional to the pressure of that gas above the surface of the solution.
The soda is flat there is little or no carbon dioxide still left in solution. Lowering the temperature of a liquid decreases. During the summer warm water has less Oxygen gas dissolved in the water.
B when the pressure is high and the temperature is high. Cold liquid dissolves more gas because gas particles are able to escape from the liquid into the surrounding air if they are quick enough have enough kinetic energy. In general solubility of a gas in water will decrease with increasing temperature.
As the temperature increases gases expand and escapes from their solvent. More gas can dissolve in cold water than in hot water. A when the pressure is low and the temperature is low.
Answer 1 of 8. The solubility of gases in the liquid is expressed in terms of the absorption coefficient. Another example is Oxygen in rivers and lakes.
But in the case of gaseous substance temperature inversely influences solubility ie. D when the pressure is high and the temperature is low. On the other hand the solubility of a gas in a liquid increases with the pressure exerted.
Colder water will be able to have more gas dissolved in it. Henrys Law states that. At a constant temperature the amount of a given gas that dissolves in a given type and volume of liquid is directly proportional to the partial pressure of that gas in equilibrium with that liquid Basically pressure allows gases to dissolve into liquids.
A gas is most soluble in water under conditions of high pressure and low temperature. Chemistry 12102019 1620 castellon67. Solution since this will best relieve the pressure that has been applied.
If under enough pressure all gases can dissolve in liquids. Hotter solutions of liquid and gas have more kinetic energy so the gas particles can. Its no different from any other dissolved substance.
Generally water dissolves solutes at 20 C or 100 C. Melting also called fusion is the process where solid becomes liquid sublimation is the process where solid becomes gaseous without first changing to liquid deposition involves change from gaseous to solid state freezing involves change from liquid to solid boiling or evaporation involves change of liquid to gaseous state while condensation. The volume of the gas is measured at STP.
Under which set of conditions will the unknown gas XY be most soluble in a liquid. But these molecules can get trapped in a liquid which means they hav. Dissolved in solution has increased as shown in the.
Correct answer to the question Need help asap please Under which conditions will gases best dissolve in liquids. What are the best conditions for dissolving a gas in a liquid. Quoting directly from wikipedia.
The number of gas molecules is decreased. A gas dissolves faster in a liquid if the temperature of the liquid is increased decreased. C when the pressure is low and the temperature is high.
The absorption coefficient is defined as the volume of gas in mL that can be dissolved by 1 mL of a liquid solvent at the temperature of the experiment at one atmospheric pressure. When the pressure is low and the temperature is low B. The kind of solute that dissolves best under high temperature conditions would be a solid What kind of solute solid liquid or gas dissolves best under high temperature conditions.
See full answer below. When the pressure is high and the temperature is high C. If a solid solute is not dissolving well in water you can increase its.
Sparingly soluble solid or liquid substances can be dissolved completely by increasing the temperature. In the case of gases the decrease in intermolecular forces releases the gas molecules from the forces that keep them in solution and will escape from the container so it will be observed that as the temperature increases the solubility of a gas decreases in a solvent like water. Decreased A gass solubility is faster in a liquid when under high low pressure.
In the gaseous state its molecules move about quite freely filling its container or in the case of the atmosphere moving throughout it. This can occur according to Henrys law.
13 3 Pressure And Temperature Effects On Solubility Chemistry Libretexts
Mixtures Of Liquids And Gases Henry S Law

Dissolved Gases Important Gases 6 Important Gases Are Dissolved In Lakes Streams Seas 6 Important Gases Are Dissolved In Lakes Streams Seas Nitrogen Ppt Download



Comments
Post a Comment